|
254. Nickel-Catalyzed Asymmetric α-Alkenylations of Acyclic Amides that Provide Tertiary Stereocenters
Hossain, A.; Fu, G. C. J. Am. Chem. Soc. 2025, xxx, xxxxx–xxxxx. |
|
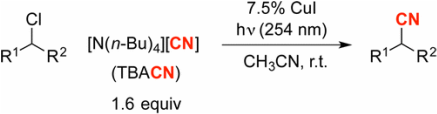
253. Photoinduced, Copper-Catalyzed Enantioconvergent Azidation of Alkyl Halides
Zhong, F.; Anderson, R. L.; Oyala, P. H.; Fu, G. C. J. Am. Chem. Soc. 2025, 147, 32963–32970. |
|
252. Photoinduced copper-catalysed deracemization of alkyl halides
Zhong, F.*; Li, R.*; Mai, B. K.; Liu, P.; Fu, G. C. Nature 2025, 640, 107–113. |
|
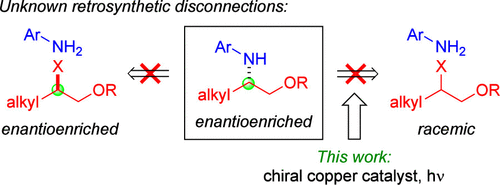
251. Photoinduced, Copper-Catalyzed Enantioconvergent Synthesis of β-Aminoalcohol Derivatives
Mondal, A.; Fu, G. C. J. Am. Chem. Soc. 2025, 13, 10859–10863. |
|
250. Synthesis of tertiary alkyl amines via photoinduced copper-catalysed nucleophilic substitution
Cho, H.; Tong, X.; Zuccarello, G.; Anderson, R.L.; Fu, G. C. Nat. Chem. 2025, 17, 271–278. |
|
249. Nickel-Catalyzed Enantioconvergent and Diastereoselective Allenylation of Alkyl Electrophiles: Simultaneous Control of Central and Axial Chirality
Hossain, A.; Anderson, R.L.; Zhang, C.S.; Chen, P.-J.; Fu, G. C. J. Am. Chem. Soc. 2024, 11, 7173–7177. |
|
248. Phosphine Catalysis of the Fluorination of Unactivated Tertiary Alkyl Chlorides under Mild and Convenient Conditions
Wang, Z.-Y.; Freas, D.J.; Fu, G. C. J. Am. Chem. Soc. 2023, 46, 25093–25097. |
|
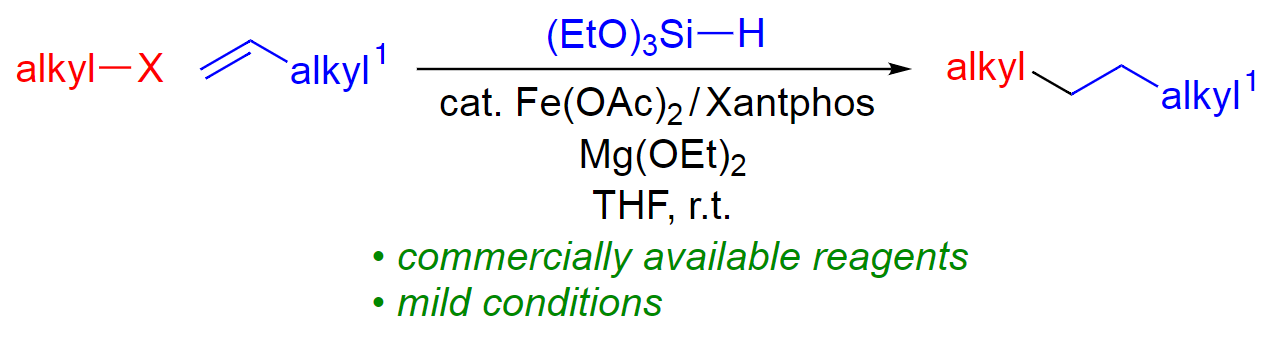
247. Iron-Catalyzed Reductive Cross-Coupling of Alkyl Electrophiles with Olefins
Tong, X.; Yang, Z.; Del Angel Aguillar, C. E.; Fu, G. C. Angew. Chem. Int. Ed. 2023, 62, e202306663. |
|
246. Copper-Catalyzed Enantioconvergent Alkylation of Oxygen Nucleophiles
Chen, C.; Fu, G. C. Nature 2023, 618, 301–307. |
|
245. Enantioselective Synthesis of α-Aminoboronic Acid Derivatives via Copper-Catalyzed N-Alkylation
Zuccarello, G.; Batiste, S. M.; Cho, H.; Fu, G. C. J. Am. Chem. Soc. 2023, 145, 3330–3334. |
|
244. Catalytic Enantioselective α-Alkylation of Amides by Unactivated Alkyl Electrophiles
Tong, X.; Schneck, F.; Fu, G. C. J. Am. Chem. Soc. 2022, 144, 14856–14863. |
|
243. Photoinduced, Copper-Catalyzed Enantioconvergent Alkylations of Anilines by Racemic Tertiary Electrophiles: Synthesis and Mechanism
Cho, H.; Suematsu, H.; Oyala, P. H.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc. 2022, 144, 4550–4558. |
|
242. Investigation of the C–N Bond-Forming Step in a Photoinduced, Copper-Catalyzed Enantioconvergent N–Alkylation: Characterization and Application of a Stabilized Organic Radical as a Mechanistic Probe
Lee, H.; Ahn, J. M.; Oyala, P. H.; Citek, C.; Yin, H.; Fu, G. C.; Peters, J. C. J. Am. Chem. Soc. 2022, 144, 4114–4123. |
|
241. Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity
Chen, C.; Peters, J. C.; Fu, G. C. Nature 2021, 596, 250–256. |
|
240. Asymmetric Synthesis of Protected Unnatural α-Amino Acids via Enantioconvergent Nickel-Catalyzed Cross-Coupling
Yang, Z.-P.*; Freas, D. J.*; Fu, G. C. J. Am. Chem. Soc. 2021, 143, 8614–8618. |
|
239. The Asymmetric Synthesis of Amines via Nickel-Catalyzed Enantioconvergent Substitution Reactions
Yang, Z.-P.*; Freas, D. J.*; Fu, G. C. J. Am. Chem. Soc. 2021, 143, 2930–2937. |
|
238. Quaternary stereocentres via catalytic enantioconvergent nucleophilic substitution reactions of tertiary alkyl halides
Wang, Z.; Yang, Z.-P.; Fu, G. C. Nat. Chem. 2021, 13, 236–242. |
|
237. Convergent Catalytic Asymmetric Synthesis of Esters of Chiral Dialkyl Carbinols
Yang, Z.-P.; Fu, G. C. J. Am. Chem. Soc. 2020, 142, 5870–5875. |
|
236. Catalyst-controlled doubly enantioconvergent coupling of racemic alkyl nucleophiles and electrophiles
Huo, H.; Gorsline, B. J.; Fu, G. C. Science 2020, 367, 559–564. |
|
235. Mechanistic Investigation of Enantioconvergent Kumada Reactions of Racemic α-Bromoketones Catalyzed by a Nickel/Bis(oxazoline) Complex
Yin, H.; Fu, G. C. J. Am. Chem. Soc. 2019, 141, 15433–15440. |
|
234. Enantioconvergent Alkylations of Amines by Alkyl Electrophiles: Copper-Catalyzed Nucleophilic Substitutions of Racemic α-Halolactams by Indoles
Bartoszewicz, A.; Matier, C. D.; Fu, G. C. J. Am. Chem. Soc. 2019, 141, 14864–14869. |
|
233. Enantioconvergent Cross-Couplings of Alkyl Electrophiles: The Catalytic Asymmetric Synthesis of Organosilanes
Schwarzwalder, G. M.; Matier, C. D.; Fu, G. C. Angew. Chem. Int. Ed., 2019, 58, 3571–3574. |
|
232. Visible-Light-Induced, Copper-Catalyzed Three-Component Coupling of Alkyl Halides, Olefins, and Trifluoromethylthiolate To Generate Trifluoromethyl Thioethers
He, J.; Chen, C.; Fu, G. C.; Peters, J. C. ACS Catal., 2018, 8, 11741–11748. |
|
231. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins
Wang, Z.; Yin, H.; Fu, G. C. Nature, 2018, 563, 379–383. |
|
230. Nickel-Catalyzed Enantioconvergent Borylation of Racemic Secondary Benzylic Electrophiles
Wang, Z.; Bachman, S.; Dudnik, A. S.; Fu, G. C. Angew. Chem. Int. Ed., 2018, 57, 1–5. |
|
229. Design of a Photoredox Catalyst that Enables the Direct Synthesis of Carbamate-Protected Primary Amines via Photoinduced, Copper-Catalyzed N-Alkylation Reactions of Unactivated Secondary Halides
Ahn, J. M.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc., 2017, 139, 18101–18106. |
|
228. Copper-Catalyzed Alkylation of Aliphatic Amines Induced by Visible Light
Matier, C. D.; Schwaben, J.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc., 2017, 139, 17707–17710. |
|
227. Photoinduced,Copper-Catalyzed Decarboxylative C–N Coupling to Generate Protected Amines: An Alternative to the Curtius Rearrangement
Zhao, W.; Wurz, R. P.; Peters, J. P.; Fu, G. C. J. Am. Chem. Soc., 2017, 139, 12153–12156. |
|
226. Photoinduced,Copper-Catalyzed Alkylation of Amines: A Mechanistic Study of the Cross-Coupling of Carbazole with Alkyl Bromides
Ahn, J. M.; Ratani, T. S.; Hannoun, K. I.; Fu, G. C.; Peters, J. C. J. Am. Chem. Soc., 2017, 139, 12716–12723. |
|
225. Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes
Fu, G. C. ACS Cent. Sci., 2017, 3, 692–700. |
|
224. Control of Vicinal Stereocenters through Nickel-Catalyzed Alkyl-Alkyl Cross-Coupling
Mu, X.; Shibata, Y.; Makida, Y.; Fu, G. C. Angew. Chem. Int. Ed., 2017, 56, 5821–5824. |
223. Transition metal-catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry
Choi, J.; Fu, G. C.
Science, 2017, 356, 152–160.
Choi, J.; Fu, G. C.
Science, 2017, 356, 152–160.
|
222. Caution in the Use of Nonlinear Effects as a Mechanistic Tool for Catalytic Enantioconvergent Reactions: Intrinsic Negative Nonlinear Effects in the Absence of Higher-Order Species
Kalek, M.; Fu, G. C. J. Am. Chem. Soc., 2017, 139, 4225–4229. |
|
221. A general, modular method for the catalytic asymmetric synthesis of alkylboronate esters
Schmidt, J.; Choi, J.; Liu, A. T.; Slusarczyk, M.; Fu, G. C. Science, 2016, 354, 1265–1269. |
|
220. Catalytic Enantioselective Carbon–Oxygen Bond Formation: Phosphine-Catalyzed Synthesis of Benzylic Ethers via the Oxidation of Benzylic C–H Bonds
Ziegler, D. T.; Fu, G. C. J. Am. Chem. Soc., 2016, 138, 12069–12072. |
|
219. Silicon–Carbon Bond Formation via Nickel-Catalyzed Cross-Coupling of Silicon Nucleophiles with Unactivated Secondary and Tertiary Alkyl Electrophiles
Chu, C. K.; Liang, Y.; Fu, G. C. J. Am. Chem. Soc., 2016, 138, 6404–6407. |
218. A mechanistic investigation of the photoinduced, copper-mediated cross-coupling of an aryl thiol with an aryl halide
Johnson, M. W.; Hannoun, K., I.; Tan, Y.; Fu, G. C.; Peters, J. C.
Chem. Sci., 2016, 7, 4091–4100.
Johnson, M. W.; Hannoun, K., I.; Tan, Y.; Fu, G. C.; Peters, J. C.
Chem. Sci., 2016, 7, 4091–4100.
|
217. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light
Kainz, Q. M.; Matier, C. D.; Bartoszewicz, A.; Zultanski, S. L.; Peters, J. C.; Fu, G. C. Science 2016, 351, 681–684. |
|
216. Enantioselective Decarboxylative Arylation of α-Amino Acids via the Merger of Photoredox and Nickel Catalysis
Zuo, Z; Cong, H.; Li, W.; Choi, J.; Fu, G. C.; MacMillan, D. W. C. J. Am. Chem. Soc. 2016, 138, 1832–1835. |
|
215. Photoinduced, Copper-Catalyzed Carbon–Carbon Bond Formation with Alkyl Electrophiles: Cyanation of Unactivated Secondary Alkyl Chlorides at Room Temperature
Ratani, T. S.; Bachman, S.; Fu, G. C.; Peters, J. C. J. Am. Chem. Soc. 2015, 137, 13902–13907. |
|
214. Stereoconvergent Negishi Arylations of Racemic Secondary Alkyl Electrophiles: Differentiating between a CF3 and an Alkyl Group
Liang, Y.; Fu, G. C. J. Am. Chem. Soc. 2015, 137, 9523–9526. |
|
213. Phosphine-Catalyzed Doubly Stereoconvergent γ-Additions of Racemic Heterocycles to Racemic Allenoates: The Catalytic Enantioselective Synthesis of Protected α,α-Disubstituted α-Amino Acid Derivatives
Kalek, M.; Fu, G. C. J. Am. Chem. Soc. 2015, 137, 9438–9442. |
|
212. Nickel-Catalyzed Alkyl–Alkyl Cross-Couplings of Fluorinated Secondary Electrophiles: A General Approach to the Synthesis of Compounds having a Perfluoroalkyl Substituent
Liang, Y.; Fu, G. C. Angew. Chem. Int. Ed. 2015, 54, 9047–9051. |
|
211. Phosphine-Catalyzed Enantioselective Intramolecular [3+2] Annulations To Generate Fused Ring Systems
Lee, S. Y.; Fujiwara, Y.; Nishiguchi, A.; Kalek, M.; Fu, G. C. J. Am. Chem. Soc. 2015, 137, 4587–4591. |
|
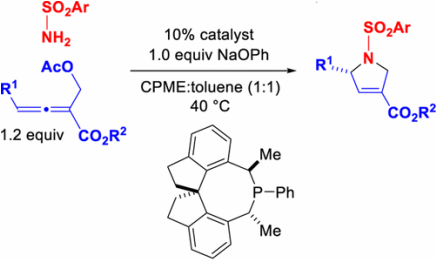
210. Use of a New Spirophosphine To Achieve Catalytic Enantioselective
[4 + 1] Annulations of Amines with Allenes To Generate Dihydropyrroles Kramer, S.; Fu, G. C. J. Am. Chem. Soc. 2015, 137, 3803–3806. |